Translate this page into:
Intraluminal Migration of Pelvic Drain Following Palliative Colonic Resection: A Rare but Risky Complication
*Corresponding author: Thejashwini S I, Department of General Surgery, Sri Dharmasthala Manjunatheshwara Medical College and Hospital, Dharwad, India theju241si@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Thejashwini SI, Pai S, Patil VA. Intraluminal Migration of Pelvic Drain Following Palliative Colonic Resection: A Rare but Risky Complication. Karnataka J Surg. 2025;2:21–24. doi: 10.25259/KJS_18_2024
Abstract
Enhanced recovery after surgery (ERAS) protocols discourage drains after colorectal surgery as there are potential complications. However, sometimes, a drain is placed based on the surgeon’s decision. Our report highlights a case of carcinoma rectosigmoid; in his late sixties, he underwent palliative resection due to intestinal obstruction. We noticed faecal discharge in the drain, which gradually reduced. On per-rectal examination on day 15, we could palpate the drain. An emergency laparotomy was done, and we found that the drain had migrated through the anastomotic line and entered the lumen of the rectum. The drain was removed, and the breach in the anastomotic line was closed primarily, and a diversion ileostomy was performed. Discretion is needed before drain placement during surgery as there is a potential risk of migration of the drain through the anastomotic line. The aim of this report is to highlight this risk.
Keywords
Anastomotic leak
Colorectal neoplasms
Colorectal surgery
Fistula
Postoperative complications
INTRODUCTION
The use of intraperitoneal drains in colorectal surgery is controversial, but the practice still prevails. Evidence does not support this practice following colorectal surgery, but there is insufficient data to determine whether drains influence the rates of anastomotic leak.[1,2] In contrast, drain placements are the standard treatment for patients with intra-abdominal abscesses and selected cases of anastomotic leaks.[3–5] The topic of intra-abdominal drain migration has received less attention, but data suggests that it is relatively common, affecting approximately 19.8% of patients undergoing digestive abdominal surgery.[6]
We present a case of a patient in his late 60s with stage 4 carcinoma of the rectosigmoid junction who underwent palliative resection with a pelvic drain placed. Despite initial management of a low-output anastomotic leak, gradual improvement misled us into a false sense of recovery until a per-rectal examination on day 15 revealed a drain in the rectum. Emergency relaparotomy confirmed an anastomotic leak caused by the drain entering the rectal lumen. The leak was closed primarily, and a diversion loop ileostomy was performed.
This rare case underscores the risks associated with postoperative drains, including anastomotic failure and subsequent fistula development. We aim to highlight the risks involved with postoperative drains, including contributing to anastomotic failure and subsequent fistula development, hence highlighting the need for vigilance in drain management as a crucial step to prevent such complications in colorectal surgery.
CASE REPORT
Our patient, in his late 60s, presented with a history of constipation for 2 weeks. The patient was evaluated at an outside hospital 2 days prior to presentation at our hospital with a contrast-enhanced computed tomography scan (CECT) of the abdomen and pelvis reported as carcinoma rectosigmoid junction and proximal rectum with liver metastasis. A sigmoidoscopy done at an outside centre reported a luminal occlusion at 12 cm from the anal verge; a biopsy suggested adenocarcinoma. He presented to us with an acute large bowel obstruction of 2 days. On examination, he had no pallor or icterus. His abdomen was distended with diffuse tenderness with guarding in the lower abdomen. There was no liver or splenic enlargement, and no mass was palpable. The per-rectal examination was normal. The patient was diagnosed with a large bowel obstruction secondary to the rectosigmoid growth. We considered a diversion colostomy, as well as Hartmann’s procedure, but when the patient underwent emergency exploratory laparotomy, primary resection was feasible, so we performed resection of the sigmoid colon and upper 1/3rd of the rectum with side-to-end colorectal anastomosis. We placed a 32F chest tube as a passive drain. On post operative day 4 nasogastric tube was removed and patient was allowed orally, and patient passed stools on post operative day 5. On postoperative day 5, we noticed a feculent discharge in the intra-abdominal pelvic drain, with a volume of around 150–200 ml. We performed a CECT abdomen; this confirmed a low-output anastomotic leak.
The drain was not removed, and he continued to be fed orally, and gradually the drain output was reduced to 5 ml. He also started passing well-formed stools. However, on postoperative days 14 and 15, he abruptly stopped passing stools. On per rectal examination, the drain tube was palpable, which was confirmed on proctoscopy. Hence, the patient was diagnosed to have intra-luminal drain tube migration and was posted for re-exploratory laparotomy. It was discovered that it was not an anastomotic leak and that the drain had migrated through the anastomotic line and entered the lumen of the rectum. There was a fistulous tract around the pelvic intra-abdominal drain and small bowel loops extending to the anastomotic site with a drain tip felt in the rectum per rectal examination intraoperatively [Figure 1]. The drain was removed, the fistulous tract [Figure 2] was excised, an anastomotic breach was closed, and a diversion loop ileostomy was done. A colostomy was not attempted for fear of disrupting the entire anastomosis. The patient started on an oral diet on postoperative day 1 and was discharged on postoperative day 7 with a healthy and functional stoma.
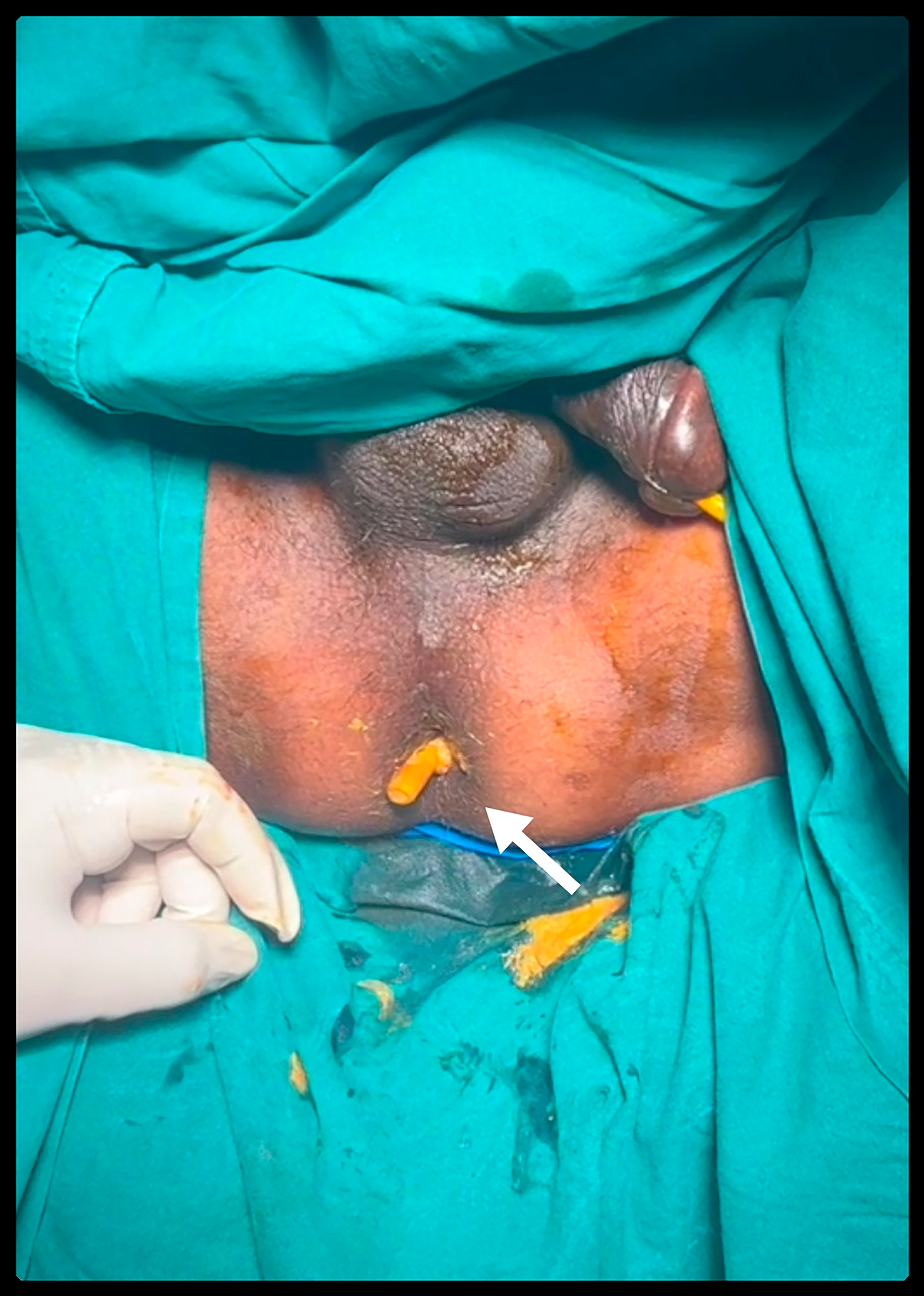
- Arrow mark shows the intra-abdominal drain tip at the anal verge.
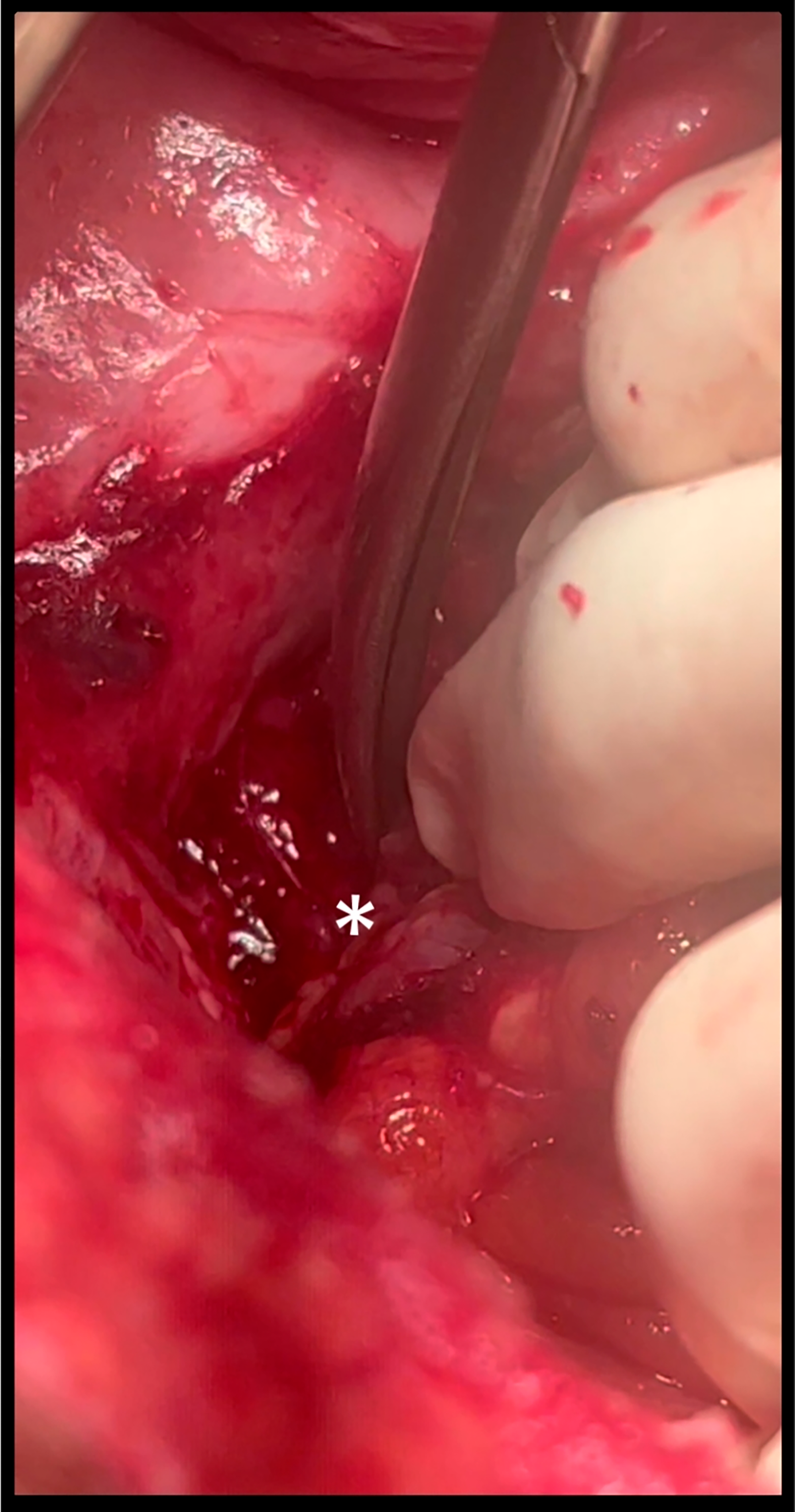
- Asterisk shows anastomosis leak site with fistulous tract.
Outcome and follow-up
The patient recovered and was tolerating a regular oral diet with a functional and healthy stoma. He was discharged on the 7th postoperative day, with the biopsy of the rectosigmoid growth reported as well-differentiated adenocarcinoma (pT4aN1bM1-stage IV) with negative proximal and distal margins of the tumour [Figure 3]. We planned for ileostomy closure after 6 weeks, followed by palliative chemotherapy, but the patient presented after 12 weeks. We performed a colonoscopy before ileostomy closure and found that he had developed ulceroproliferative growth at the anastomotic site. The biopsy revealed an adenocarcinoma [Figure 4]. We deferred the closure in favour of palliative chemotherapy. He wanted time to decide and succumbed to his ailment a few weeks later.

- Rectosigmoidectomy specimen biopsy report—well-differentiated adenocarcinoma (pT4aN1bM1-stage IV) with negative proximal and distal margins of the tumour.

- Colonoscopic biopsy report of anastomotic site—well-differentiated adenocarcinoma.
DISCUSSION
The use of a drain following colonic surgeries is a controversial subject amongst 21st-century surgeons. Most protocols and guidelines discourage this practice.[7,8] The dictum of Lawson Tait, “When in doubt, drain,” guided surgeons for generations,[1] which gradually evolved to “When in doubt, do not drain.”[2] Despite this significant number of surgeons still practicing the method of prophylactic drain placement, however, this is plagued by complications: sepsis, injury to blood vessels, and adhesions.[3] One such complication encountered is drain migration; although uncommon, it has disastrous consequences.[4,5] Compared to colorectal surgery, the incidence of drain migration is higher in upper gastrointestinal surgeries.[4,5] The reasons for drain migration are not clear.[4,6] However, in the case of negative suction drains such as vacuum/aspiration drains, it is postulated that the drain may adhere to the bowel wall, inducing a necrotic process that culminates in bowel wall perforation and subsequent penetration of the drain into the lumen as proposed by Nomura et al.[9] The mechanism underlying the intraluminal migration of passive drains remains uncertain.[5] Potential causes for drain migration include improper fixation of the drain, suture material cutting through, low intra-abdominal pressure, and pressure exerted on the drain by the patient’s body weight when lying on the same side.[6] A. Gilbert et al. reported that abdominal drains migrate frequently, affecting up to 28% of patients and 19.8% of all drains. Interestingly, the study found no statistically significant association between the type of drain, number of drains, position, trajectory, morphology of patients, or type of intervention, and the risk of migration. Factors such as device flexibility, intra-abdominal visceral peristalsis, and changes in patient position were implicated in drain displacement.[4] Compared to laparotomy (25.3%), laparoscopically placed drains exhibited a higher tendency to migrate (38.5%). Emergency surgeries (37.5%) showed a higher migration rate compared to elective surgeries (25.7%). The study recommends intraperitoneal fixation of drains to mitigate migration risk.[4] Surgeons often place prophylactic drains at anastomotic sites; persistent drainage frequently serves as the initial indication of anastomotic leakage or drain migration. Diagnosis of anastomotic leak or drain migration typically involves contrast radiographic studies, where evidence of leakage post-radio-contrast examination suggests either anastomotic leakage or drain migration. The endoscopic examination can further confirm the diagnosis.[5] While data support non-operative management of drain migration, it is imperative to rule out fistula associated with migration before considering non-operative approaches.[10]
Learning points/take home messages
It is important to have a differential of possible intraluminal drain migration in case of persistent or increased drain output.
To drain or not is the surgeon’s choice, as it has no impact on the outcome.
Management of migrated drain can be non-operative or operative.
It is important to rule out the possibility of a fistula that can be formed with the migration.
CONCLUSION
Usage of drains is not without complications, and drain migration, though not common, can lead to catastrophic complications; hence, it is important to have a differential of possible intraluminal drain migration in case of persistent or increased drain output, and it is also important to rule out the possibility of a fistula that can be formed with the migrated drain. The clinical importance of drain migration appears to be minimal, and the decision whether to use a drain following colorectal surgery should be made on a case-by-case basis, taking into account factors such as the surgeon’s judgement and patient preferences. Ultimately, the decision to drain or not to drain should prioritise patient safety and optimal postoperative outcomes.
Author contributions
TSI: Patient care, Literature search, data acquisition, manuscript preparation, editing and review; SP: Photographs, patient care, guidance in literature research; VAP: Operating surgeon.
Ethical approval
Institutional Review Board approval is not required.
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
REFERENCES
- Enhanced Recovery After Surgery for Emergency Colorectal Surgery: Are There any Differences Between Intra-Abdominal Infection and Other Indications? J Visc Surg [Internet]. 2019;156:489-96.
- [Google Scholar]
- Complications of Colorectal Anastomoses: Leaks, Strictures, and Bleeding. Surg Clin N Am. 2013;93:61-87.
- [CrossRef] [PubMed] [Google Scholar]
- Intraluminal Migration of a Penrose Drain Presented With Hematochezia, After Lower Gastrointestinal Surgery. Surg J (N Y). 2022;8:e279-82.
- [PubMed] [PubMed Central] [Google Scholar]
- Lesson Learnt from a Migrated Drain: A Case Report. Ann Med Surg (Lond). 2017;20:80-3.
- [Google Scholar]
- Implementing Enhanced Perioperative Care in Emergency General Surgery: A Prospective Multicenter Observational Study. World J Surg [Internet]. 2023;47:1339-47.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- The Factors Related to Failure of Enhanced Recovery After Surgery (ERAS) in Colon Cancer Surgery. Langenbecks Arch Surg [Internet]. 2020;405:1025-30.
- [CrossRef] [PubMed] [Google Scholar]
- Bowel Perforation Caused by Silicone Drains: A Report of Two Cases. Surg Today. 1998;28:940-2.
- [Google Scholar]
- Percutaneous Postoperative Intra-Abdominal Abscess Drainage After Elective Colorectal Surgery. Tech Coloproctol. 2002;6:159-64.
- [CrossRef] [PubMed] [Google Scholar]








